Noble Metal Electrocatalysts: Benefits of Chemical Functionalization | CPT PPP Coverage
Cryptopolytech (CPT) Public Press Pass (PPP)
News of the Day COVERAGE
200000048 – World Newser
•| #World |•| #Online |•| #Media |•| #Outlet |
View more Headlines & Breaking News here, as covered by cryptopolytech.com
Noble Metal Electrocatalysts: Benefits of Chemical Functionalization appeared on www.azonano.com by .
Electrocatalysis is an interface-dominated process in which the catalyst activity is highly related to the adsorption or desorption behaviors of the intermediates or reactants, or products on the active sites.
From the catalyst design point of view, the chemical functionalization on noble metal surfaces will unavoidably impact the reaction process. This is known to be one of the efficient plans to tune the noble metal nanocrystals’ electrocatalytic performance.
In recent times, a research group headed by Professor Yu Chen from Shaanxi Normal University, China reported a new paper in the field of noble metal electrocatalysis.
Their study outlines the synthesis techniques of polyamine (PAM) functionalized noble metal nano-electrocatalysts and also their applications in electrocatalytic reactions, and presents the research advance, present deficiencies, difficulties, and future possibilities of chemically functionalized noble metal electrocatalysts.
This study has been reported in the Chinese Journal of Catalysis.
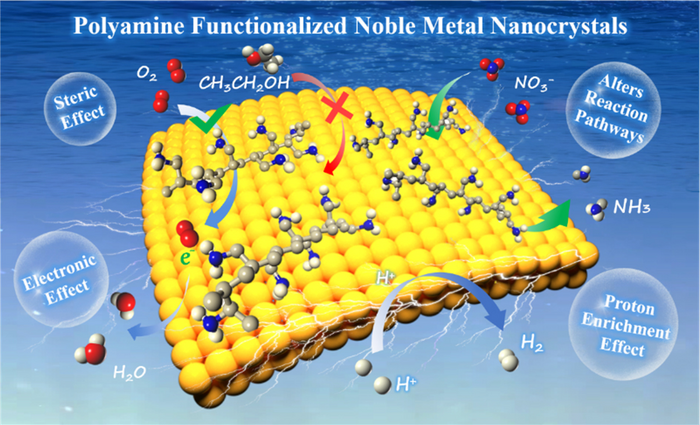
The PAM molecule’s formation mechanism functionalized noble metal nanocrystals is first discussed. The authors describe that PAM consists of a huge number of amino groups (?NH2) or imino groups (?NH?), in which the single pair of electrons on the nitrogen atom has a powerful coordination potential.
As far as the hydrothermal reaction is concerned, PAM could interact with RhIII, PtII, AgI, and PdII to develop complexes that shift the growth process of noble metal nanocrystals from thermodynamic control to kinetics control.
Under kinetic control, it is not necessary for the eventual shape of noble metal nanocrystals to develop nanospheres with minimal surface free energy, and several anisotropic nanostructures will be obtained depending on the reaction conditions, like nanowires, nanocubes, nanosheets, and also nanonetworks.
The electrocatalysts that are PAM functionalized have been employed in a few significant electrochemical reactions like oxygen reduction reaction (ORR) and hydrogen precipitation reaction (HER), which usually helps disclose improved electroactivity.
Generally, a vast amount of ?NH? and ?NH2 in PAM will be protonated to develop ?NH3+ and ?NH2+ in acidic or neutral media. This will directly result in raising the surface proton concentration of PAM-functionalized noble metal nanocrystals.
For the proton-coupled electrocatalytic reactions, like ORR and HER, PAM-functionalized noble metal nanocrystals display lower reaction overpotentials and greater catalytic efficiency as a result of the interfacial proton enrichment.
Besides, the effects of PAM functionalization (like steric hindrance effect, electronic effect, and group effect) on catalyst activity and selectivity are emphasized.
Eventually, flaws, difficulties, and views in this hopeful emerging research field are precisely outlined. This study aims to stimulate further attention to surfaces or interface functionalization and catalysis, increase investment in surfaces or interface functionalization research, and will certainly alter the future renewable energy production and environmental technologies associated with electrocatalysis.
Journal Reference:
Xue, Q., et al. (2023) Chemical functionalized noble metal nanocrystals for electrocatalysis. Chinese Journal of Catalysis. doi.org/10.1016/S1872-2067(22)64186-X.
FEATURED ‘News of the Day’, as reported by public domain newswires.
View ALL Headlines & Breaking News here.
Source Information (if available)
This article originally appeared on www.azonano.com by – sharing via newswires in the public domain, repeatedly. News articles have become eerily similar to manufacturer descriptions.
We will happily entertain any content removal requests, simply reach out to us. In the interim, please perform due diligence and place any content you deem “privileged” behind a subscription and/or paywall.
CPT (CryptoPolyTech) PPP (Public Press Pass) Coverage features stories and headlines you may not otherwise see due to the manipulation of mass media.
First to share? If share image does not populate, please close the share box & re-open or reload page to load the image, Thanks!