Safety, tolerability, and immunogenicity of the chimpanzee adenovirus type 3-vectored Marburg virus (cAd3-Marburg) vaccine in healthy adults in the USA: a first-in-human, phase 1, open-label, dose-escalation trial | CPT PPP Coverage
Cryptopolytech (CPT) Public Press Pass (PPP)
News of the Day COVERAGE
200000048 – World Newser
•| #World |•| #Online |•| #Media |•| #Outlet |
View more Headlines & Breaking News here, as covered by cryptopolytech.com
Safety, tolerability, and immunogenicity of the chimpanzee adenovirus type 3-vectored Marburg virus (cAd3-Marburg) vaccine in healthy adults in the USA: a first-in-human, phase 1, open-label, dose-escalation trial appeared on www.thelancet.com by The Lancet.
Background
(WHO)has identified Marburg virus as an emerging virus requiring urgent vaccine research
and development, particularly due to its recent emergence in Ghana. We report results
from a first-in-human clinical trial evaluating a replication-deficient recombinant
chimpanzee adenovirus type 3 (cAd3)-vectored vaccine encoding a wild-type Marburg
virus Angola glycoprotein (cAd3-Marburg) in healthy adults.
Methods
We did a first-in-human, phase 1, open-label, dose-escalation trial of the cAd3-Marburg
vaccine at the Walter Reed Army Institute of Research Clinical Trials Center in the
USA. Healthy adults aged 18–50 years were assigned to receive a single intramuscular
dose of cAd3-Marburg vaccine at either 1?×?1010 or 1?×?1011 particle units (pu). Primary safety endpoints included reactogenicity assessed for
the first 7 days and all adverse events assessed for 28 days after vaccination. Secondary
immunogenicity endpoints were assessment of binding antibody responses and T-cell
responses against the Marburg virus glycoprotein insert, and assessment of neutralising
antibody responses against the cAd3 vector 4 weeks after vaccination. This study is
registered with ClinicalTrials.gov, NCT03475056.
Findings
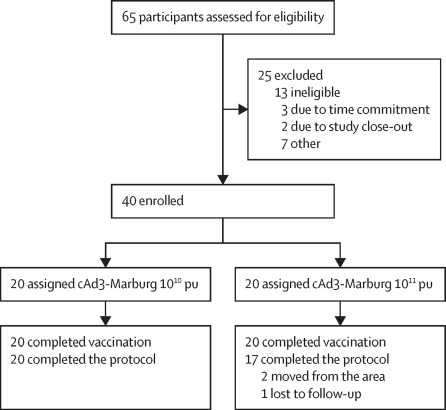
Between Oct 9, 2018, and Jan 31, 2019, 40 healthy adults were enrolled and assigned
to receive a single intramuscular dose of cAd3-Marburg vaccine at either 1?×?1010 pu (n=20) or 1?×?1011 pu (n=20). The cAd3-Marburg vaccine was safe, well tolerated, and immunogenic. All
enrolled participants received cAd3-Marburg vaccine, with 37 (93%) participants completing
follow-up visits; two (5%) participants moved from the area and one (3%) was lost
to follow-up. No serious adverse events related to vaccination occurred. Mild to moderate
reactogenicity was observed after vaccination, with symptoms of injection site pain
and tenderness (27 [68%] of 40 participants), malaise (18 [45%] of 40 participants),
headache (17 [43%] of 40 participants), and myalgia (14 [35%] of 40 participants)
most commonly reported. Glycoprotein-specific antibodies were induced in 38 (95%)
of 40 participants 4 weeks after vaccination, with geometric mean titres of 421 [95%
CI 209–846] in the 1?×?1010 pu group and 545 [276–1078] in the 1?×?1011 pu group, and remained significantly elevated at 48 weeks compared with baseline
titres (39 [95% CI 13–119] in the 1?×1010 pu group and 27 [95–156] in the 1?×1011 pu group; both p<0·0001). T-cell responses to the glycoprotein insert and neutralising
responses against the cAd3 vector were also increased at 4 weeks after vaccination.
Interpretation
This first-in-human trial of this cAd3-Marburg vaccine showed the agent is safe and
immunogenic, with a safety profile similar to previously tested cAd3-vectored filovirus
vaccines. 95% of participants produced a glycoprotein-specific antibody response at
4 weeks after a single vaccination, which remained in 70% of participants at 48 weeks.
These findings represent a crucial step in the development of a vaccine for emergency
deployment against a re-emerging pathogen that has recently expanded its reach to
new regions.
Funding
National Institutes of Health.
FEATURED ‘News of the Day’, as reported by public domain newswires.
View ALL Headlines & Breaking News here.
Source Information (if available)
This article originally appeared on www.thelancet.com by The Lancet – sharing via newswires in the public domain, repeatedly. News articles have become eerily similar to manufacturer descriptions.
We will happily entertain any content removal requests, simply reach out to us. In the interim, please perform due diligence and place any content you deem “privileged” behind a subscription and/or paywall.
CPT (CryptoPolyTech) PPP (Public Press Pass) Coverage features stories and headlines you may not otherwise see due to the manipulation of mass media.
First to share? If share image does not populate, please close the share box & re-open or reload page to load the image, Thanks!